IND application of ConVerd's first oncolytic vaccinia virus product V01 accepted
On Dec. 21, 2022, the Investigational New Drug (IND) Application of V01, the first oncolytic vaccinia virus product independently innovated by Converd, was accepted by Center For Drug Evaluation, NMPA. This is the first clinical product of the tumor system immunotherapy product developed by the ConVerd for ten years, for the treatment of various solid tumors.
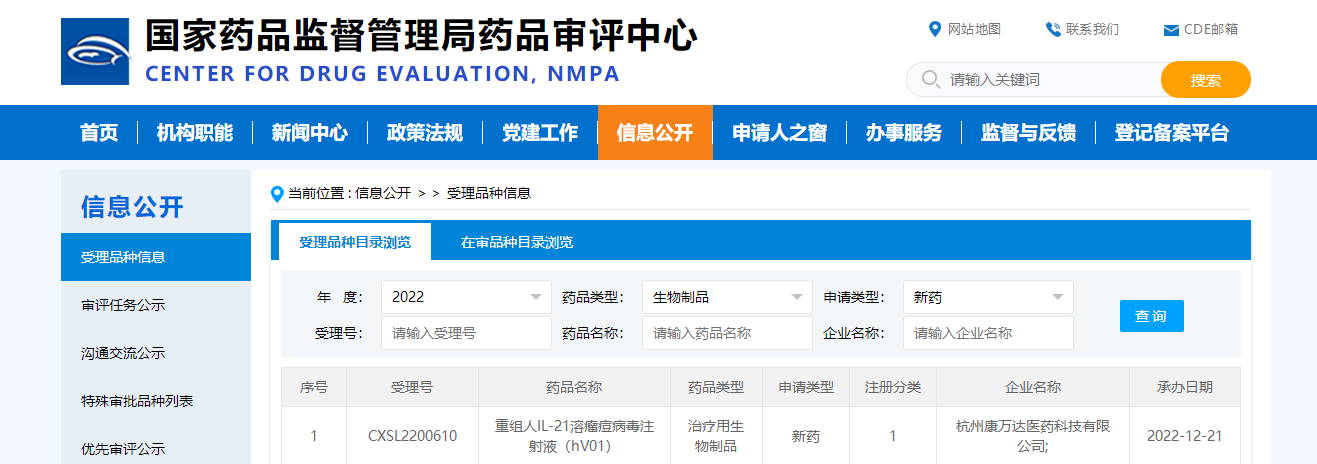
ConVerd has been committed to the development of innovative tumor systemic immunotherapies for decades. Based on years of clinical and R&D experience, it first proposed the theory of tumor system immunity and implemented it in the design of pipeline products. ConVerd has built a number of virus technology development platforms including oncolytic vaccinia virus, adenovirus, etc., integrating the industry-leading virus construction, production process development, and clinical research into one whole-process R&D system, aiming to create tumor breakthroughs in the field of oncolytic virotherapy. As of Nov. 30, 2022, ConVerd has accumulated 85 patents/patent applications, and has a complete intellectual property layout in the entire R&D process. At the same time, ConVerd is also actively looking for partners for oncolytic virus combination therapy, and strives to develop better products (combinations) for unmet clinical needs such as refractory and relapsed tumor.

